
Introduction
Manufacturing today demands more than speed and volume. It requires precision, accountability, and compliance at every stage of the product lifecycle. POMs (Points of Measure) define standards in apparel, medical device production, and other regulated industries where accuracy can mean the difference between safe and unsafe products. A POMs company ensures that measurements align with both quality expectations and international safety requirements.
When combined with PLM software and a manufacturing execution system MES, companies gain powerful capabilities: real-time oversight, process optimization, data integrity, and regulatory compliance. This combination reduces risk, enhances efficiency, and builds consumer trust, setting the stage for smarter manufacturing.
What POMs Represent in Manufacturing
POMs are not just numbers on a chart—they are critical reference points for product quality and regulatory compliance. In apparel manufacturing, they ensure correct sizing and reduce returns. In medical device production, they guarantee safety and patient well-being. In consumer packaged goods, POMs reinforce brand consistency across millions of units produced in global facilities.
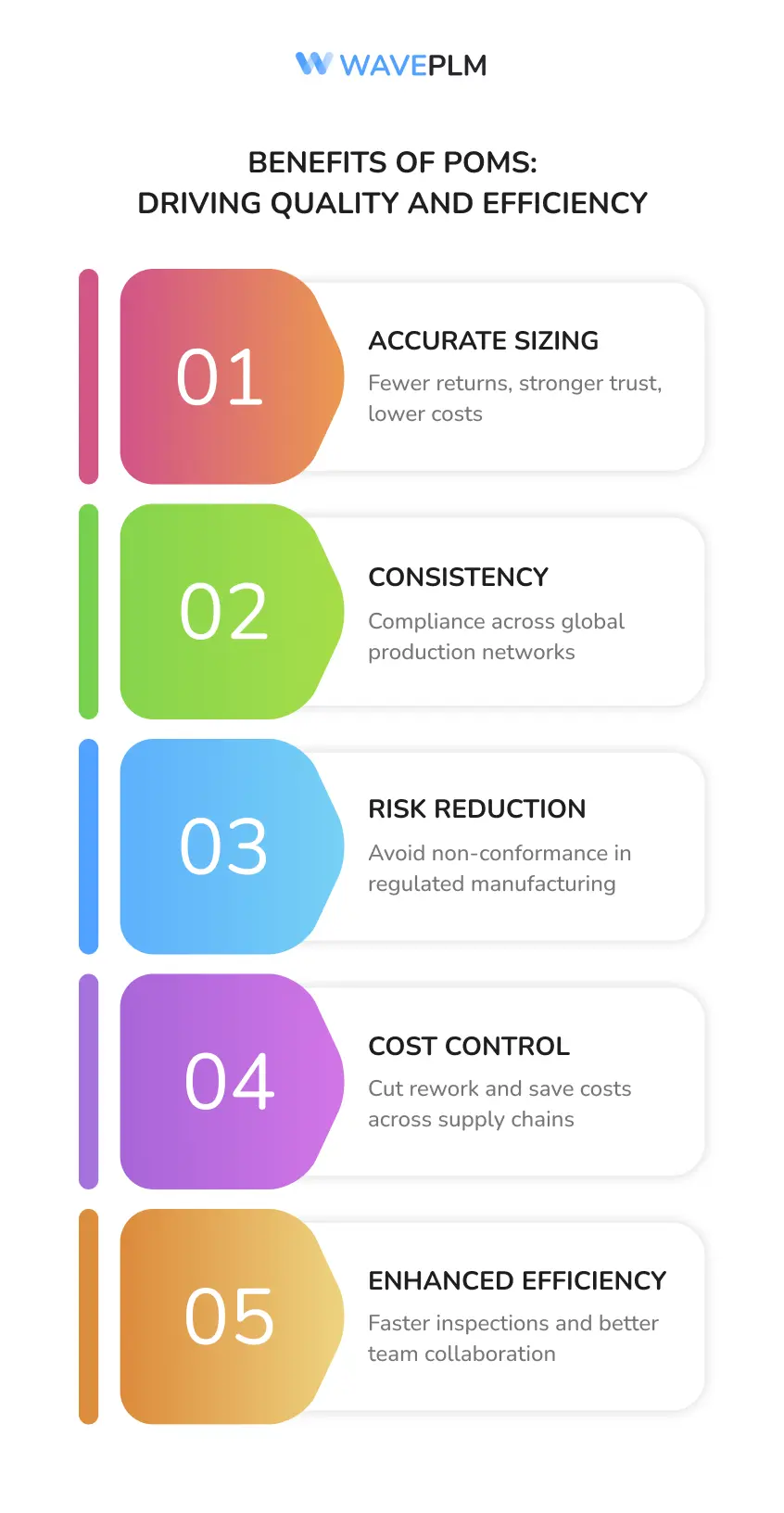
Benefits of POMs
|
Benefit |
Result |
|---|---|
|
Reduces returns, improves consumer trust, and lowers costs |
|
|
Consistency |
Ensures compliance across global production networks |
|
Risk reduction |
Prevents non-conformance in regulated manufacturers |
|
Cost control |
Minimizes rework and saves costs across supply chains |
|
Enhanced efficiency |
Speeds up inspections and improves collaboration between teams |
With POMs, regulated industries—from apparel manufacturing to gene therapy—gain a foundation for compliance, process optimization, and efficiency.
POMs and Regulatory Compliance
Regulatory compliance is central to every manufacturer’s reputation and survival. In a cGMP environment, precision reduces risk, protects consumers, and safeguards brand reputation. POMs corporation supports compliance by aligning POMs data with global standards for medical devices, consumer packaged goods, and other regulated industries.
- Medical device compliance: Exact measurement prevents failures in devices that patients rely on daily.
- Consumer packaged goods compliance: Consistent sizes and labeling accuracy improve traceability and accountability.
- Electronic batch records compliance: Digital storage of POMs ensures transparency during audits and inspections.
Using POMs solutions allows regulated manufacturers to strengthen compliance, avoid penalties, and maintain long-term client confidence.
POMs Corporation’s Role in Industry
POMs Corporation has decades of validation experience, making it a leader in regulated industries. Its configurable software empowers manufacturers to operate with precision and compliance, while also integrating with enterprise applications and other enterprise applications for seamless workflows. With its offices in South America and poms corporation herndon headquarters, the company delivers mes solutions to clients worldwide.
POMs Corporation’s Industry Knowledge
POMs Corporation’s industry knowledge ensures clients in pharma, apparel, consumer packaged goods, and gene therapy can thrive in competitive and highly regulated environments. The leadership team keeps the company well positioned to respond to emerging industry challenges while delivering proven compliance solutions.
PLM Software and MES Technology Integration
PLM software acts as the central hub for product data management. MES technology executes this data on the shop floor. When integrated seamlessly, PLM and MES create a closed loop system where POMs flow directly into production environments. This ensures that what was designed is what gets produced—without deviations.
Benefits of Integration
|
Feature |
Advantage |
|
Real-time MES data |
Prevents measurement deviations and production errors |
|
Compliance dashboards |
Strengthens regulatory compliance across regulated industries |
|
Process optimization |
Enhances efficiency across cross-functional teams |
|
Traceability |
Links electronic batch records to each unit’s product quality |
|
Cost reduction |
Identifies errors early, saving costs on rework and waste |
This integration ensures that regulated manufacturers can maintain compliance, reduce risk, and enhance efficiency while building sustainable operations.
MES Implementation and Support
MES implementation is not one-size-fits-all. Regulated manufacturers require configurable software that adapts to industry-specific demands. POMs Corporation provides mes solutions tailored to each client, helping them integrate seamlessly with business intelligence products and other enterprise applications. This support framework ensures compliance while enabling process optimization and efficiency.
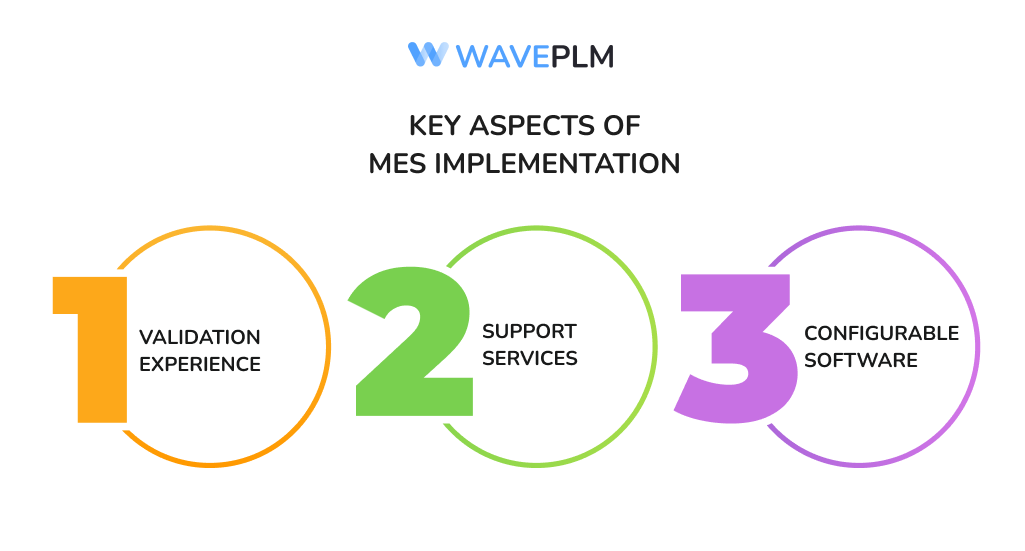
Key aspects include:
- Validation experience: Ensuring MES implementation meets global compliance standards.
- Support services: Ongoing training and system updates for clients.
- Configurable software: Flexibility for different product categories, from apparel to medical devices.
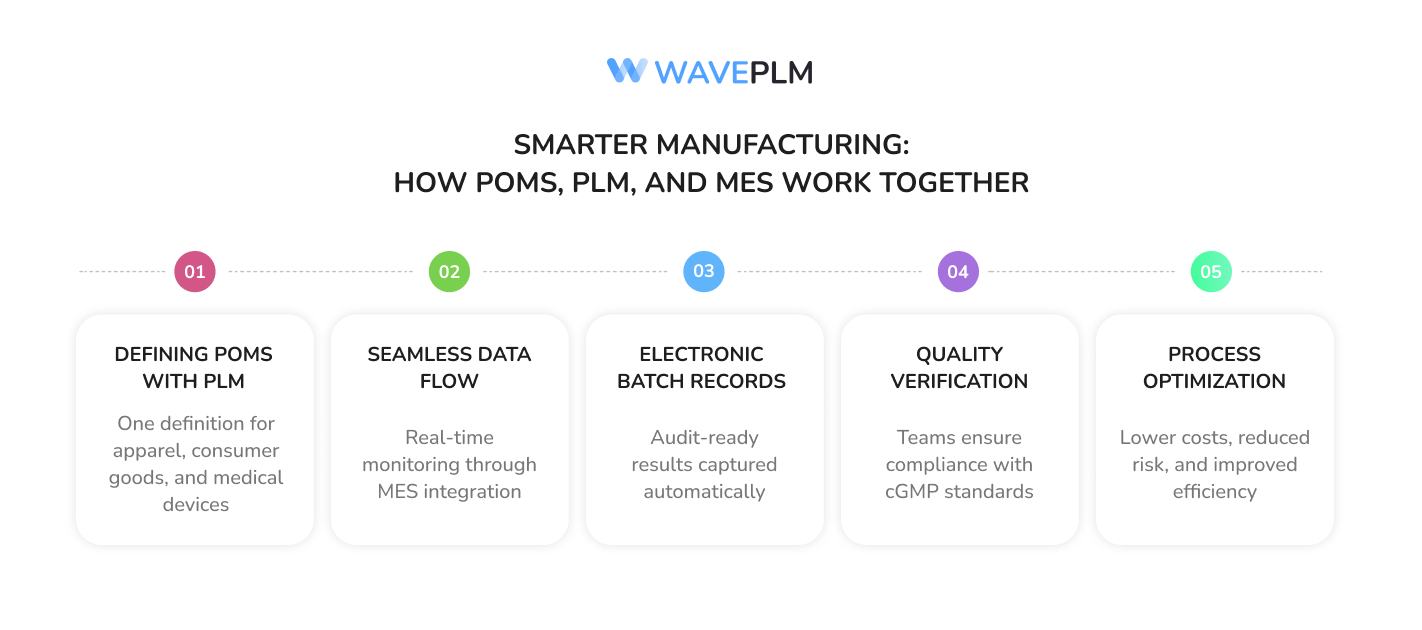
Case Example: Regulated Manufacturers in Action
- PLM defines POMs across apparel, consumer packaged goods, and medical devices.
- POMs data flows directly into the manufacturing execution system MES for real-time monitoring.
- Electronic batch records capture results, ensuring audit readiness.
- Quality control teams verify output against POMs data and cGMP environment standards.
- Process optimization reduces costs, lowers risk, and enhances efficiency across operations.
This workflow demonstrates how POMs, PLM, and MES technology integrate seamlessly to create smarter, safer, and more compliant manufacturing.
Template: POMs Compliance Workflow
|
Step |
Action |
Tool |
|
1 |
Define POMs |
|
|
2 |
Share measurement data |
PLM → MES |
|
3 |
Monitor real-time production |
MES technology |
|
4 |
Store electronic batch records |
MES + PLM integration |
|
5 |
Audit compliance |
MES dashboards + business intelligence products |
The Future of Regulated Industries
From apparel manufacturing to medical device development, POMs Corporation continues delivering proven mes solutions that reduce risk and enhance efficiency. Its presence in South America and leadership from poms corporation herndon offices reinforce its global footprint. By combining mes technology, configurable software, and validation experience, POMs Corporation ensures clients in regulated industries stay compliant while saving costs and improving product quality.
Looking ahead, regulated manufacturers will depend on MES implementation, PLM software, and POMs corporation’s industry knowledge to navigate tighter compliance landscapes. As demand grows in sectors like gene therapy, consumer packaged goods, and medical devices, POMs Corporation remains well positioned to provide clients with tailored solutions.
Conclusion
Smarter manufacturing depends on accurate data, compliance, and efficiency. POMs Corporation’s solutions combine POMs, PLM software, and manufacturing execution system MES for regulated manufacturers around the globe. With validation experience, strong support, and configurable software ensures product quality and regulatory compliance across industries, from consumer packaged goods to gene therapy.





Leave a Reply